Fisher & Paykel Healthcare expands offering in anesthesia with the release of the Optiflow Switch™ and Optiflow Trace™
Fisher & Paykel Healthcare Corporation Limited announced today it has launched two new products developed specifically for use in anesthesia applications. These applications expand the market opportunity for Optiflow™ nasal high flow within the company’s hospital respiratory support segment.
The company has released the Optiflow Switch™ and Optiflow Trace™ nasal high flow interfaces into select markets following a number of years of research and development.
“We see an opportunity to improve outcomes for patients undergoing anesthesia and believe that these new products will contribute to our aspiration of sustainable profitable growth over the long term,” said Lewis Gradon, Managing Director and Chief Executive Officer at Fisher & Paykel Healthcare.
“Based on the existing clinical evidence and our experience to date, we estimate that the number of patients annually that could benefit from Optiflow nasal high flow during anesthesia is similar to the annual number of general respiratory patients that could benefit from Optiflow,” said Mr Gradon.
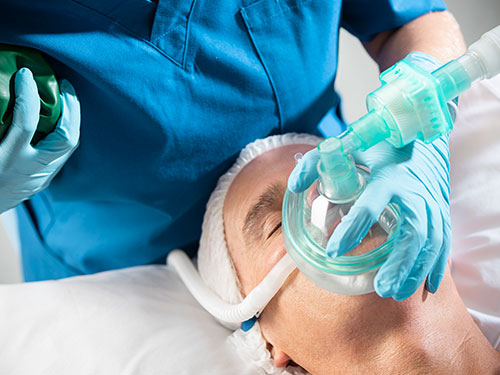
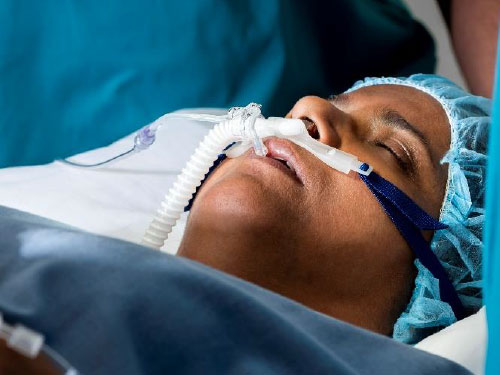
The Optiflow Switch interface, designed for general anesthesia, delivers high flows of humidified oxygen to the patient while allowing anesthesiologists to administer bag mask ventilation without needing to remove the nasal interface. The Optiflow Trace interface, used for procedural sedation, provides high flows of humidified oxygen to the patient while also allowing for the sampling of expired carbon dioxide.
“The opportunity was identified to provide a system and new range of innovative products in the anesthesia setting,” said Winston Fong, VP – Surgical Technologies for Fisher & Paykel Healthcare.
“With the new Optiflow Switch and Trace products, we are able to offer solutions to anesthesiologists right across the anesthesia care continuum,” said Mr Fong. “We have received positive feedback from medical professionals on these products, and we look forward to placing them in more markets.”
Optiflow anesthesia products are currently sold in 20 countries, including Australia, the United Kingdom, France, and the United States. Optiflow Switch will be available in the United States upon receipt of regulatory clearance.
Optiflow Switch™ Image:
Optiflow Trace™ Image:
About Fisher & Paykel Healthcare
Fisher & Paykel Fisher Healthcare is a leading designer, manufacturer and marketer of products and systems for use in acute and chronic respiratory care, surgery and the treatment of obstructive sleep apnea. The company’s products are sold in more than 120 countries worldwide. For more information about the company, visit our website www.fphcare.com.
Ends
Contact
|