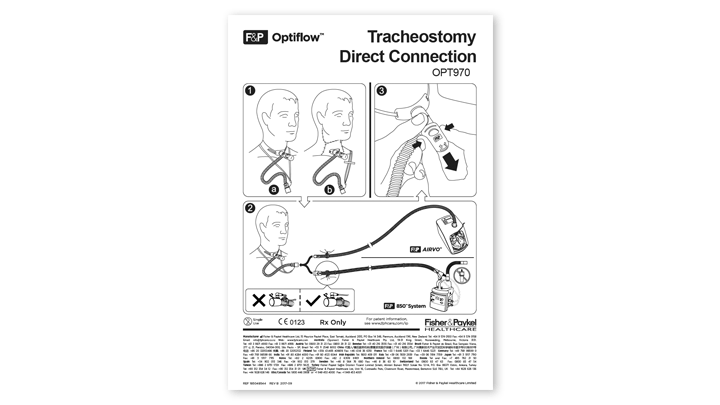
Evaqua™ technology
1. Designed to reduce the formation of condensation.
93% less condensate*
2. Connects to our AirSpiral™ breathing tube for 93% less condensate*
*compared with F&P Airvo™ 900PT501 breathing tube in internal Fisher & Paykel Healthcare testing.
Stable. Easy to use.
3. Re-designed sputum guard provides stability and
ease of use.
ease of use.
Also available as a spare part.
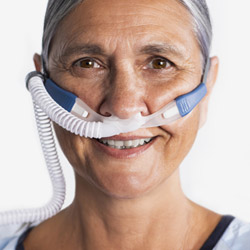
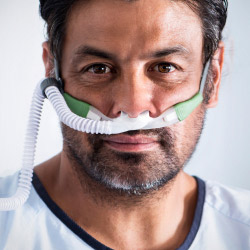
Optiflow Duet
nasal cannula interface
Delivers asymmetric nasal high flow therapy, reshaping respiratory support.
Latest NHF interface
Enquire about Optiflow nasal high flow therapy
If you have an enquiry about our products, please provide the following information so a Fisher & Paykel Healthcare representative can contact you. For further details on how this information will be used, see below or go to our privacy statement.DPM-24