What is nasal high flow (NHF/HFNC) therapy? [7 key facts to help better understand it]
1. Firstly, it’s called many things, High flow nasal cannula (HFNC), high flow therapy (HFT), high flow oxygen / therapy (HFO/T)...
2. It can deliver high levels of oxygen, but it is significantly more sophisticated than that – the higher rates of flow (independent of oxygen) confer benefits that oxygen alone cannot...
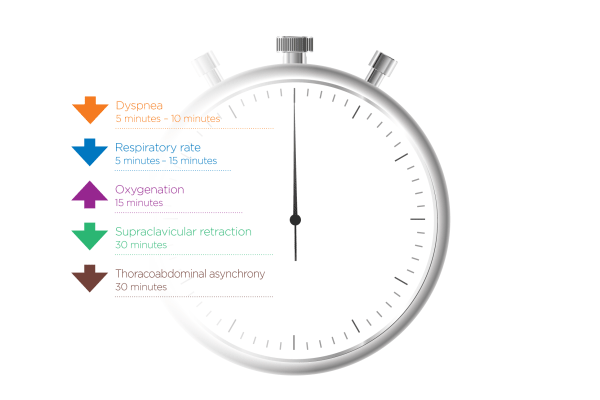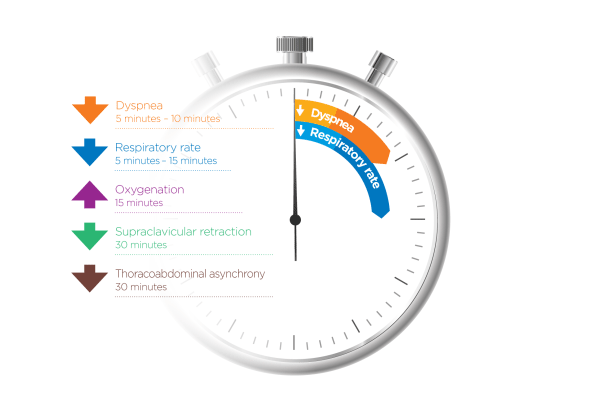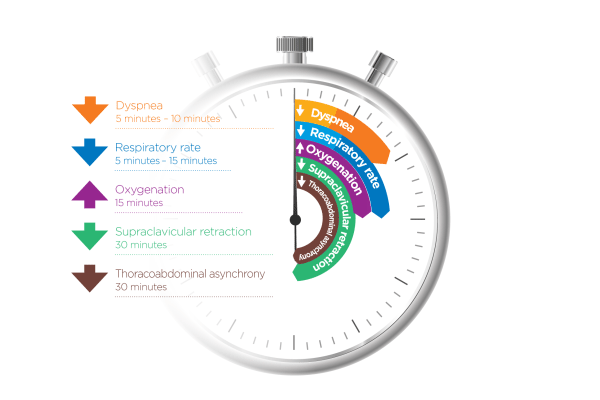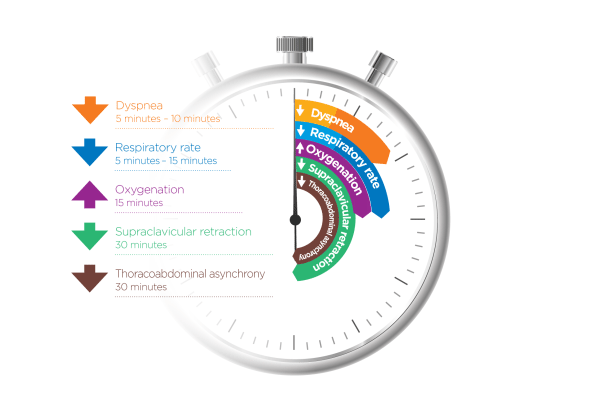
How quickly can nasal high flow work?
Evidence shows that when NHF is applied early enough (in patients who respond to therapy), you should see improvement in oxygenation, RR, and dyspnea within 15-30 mins. Monitoring should continue beyond this, of course.
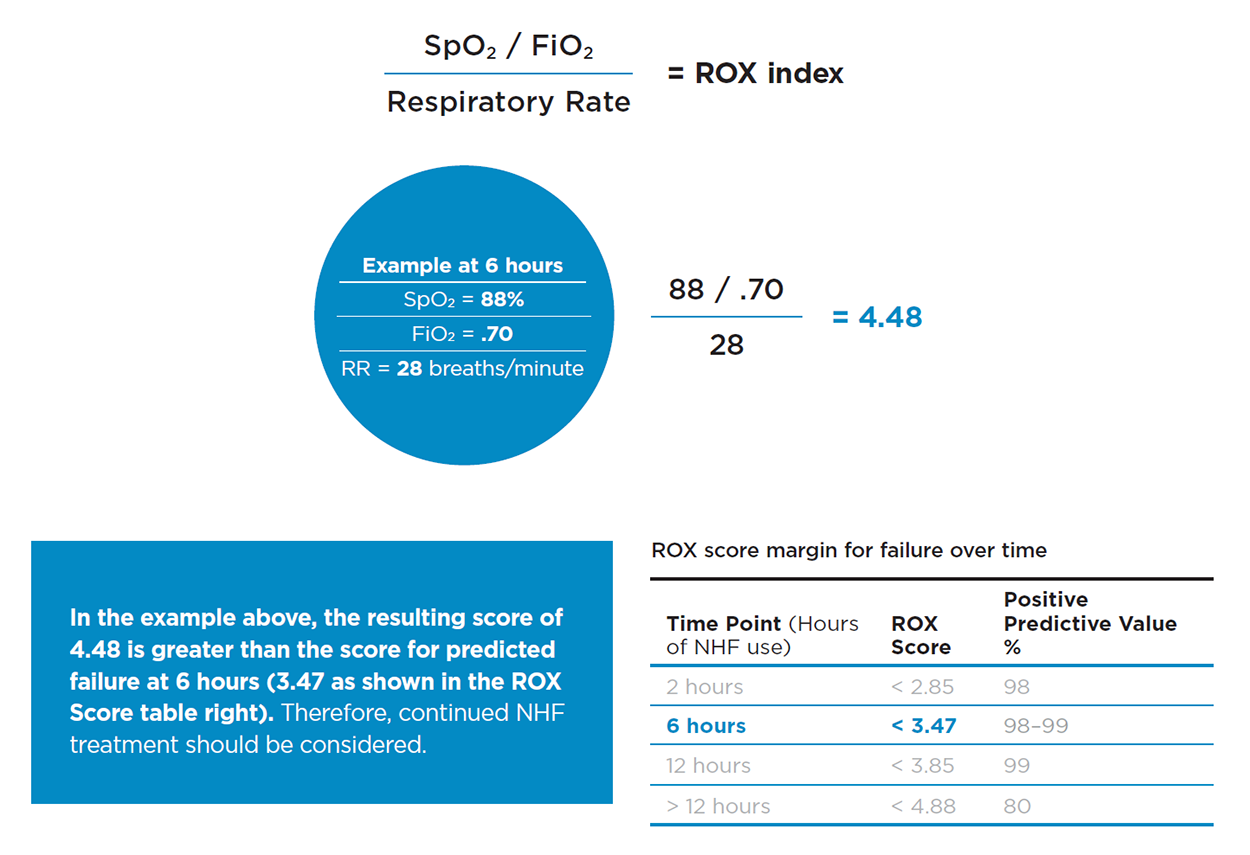
Is there a bedside tool that provides an indicator of nasal high flow outcomes? [The ROX Index]
The recent addition of the ROX index, calculated from 3 data points collected at the bedside, can provide an indicator of nasal high flow (NHF) outcome in the first few hours of treatment, and beyond.
ROX Index explained and educational tools
The ROX*index* combines three common measurements: FiO2, SpO2, and respiratory rate,...
read on...
Does nasal high flow (NHF) increase the risk of healthcare worker (HCW) infections?
This FAQ is taken from Flow Matters COVID-19 edition, updated Feb. 2022. Read the complete edition here.
Clinical observations, investigative research, and expert opinions highlight that NHF therapy is not considered to represent an increased risk of HCW infection via contact, droplet, or airborne transmission routes.
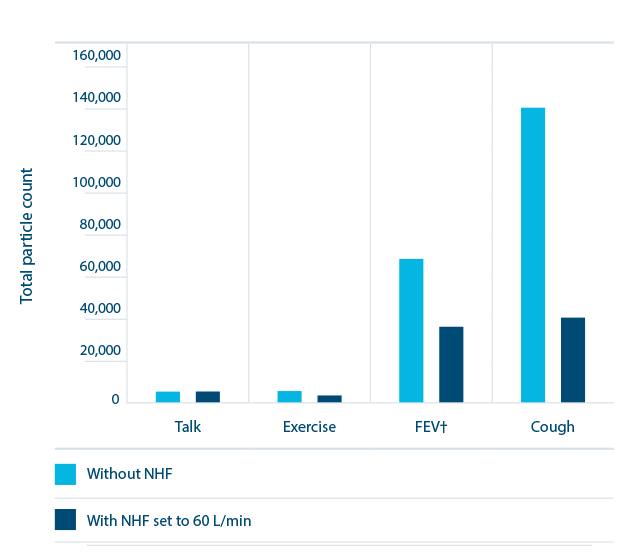
Wilson et al. 2021 compared the effect of respiratory activity, noninvasive respiratory support, and facemasks on aerosol generation.
read on....
QAre there clinical practice guidelines/recommendations for nasal high flow (aka high flow nasal cannula) therapy? UPDATED Dec 2022
QWhat is high flow oxygen?
QIs nasal high flow suitable/recommended for the treatment of COVID-19? UPDATED NOV 2021
QWhat is Optiflow nasal high flow therapy/what are my (equipment) options for delivering Optiflow therapy?
Q[The ROX Index] Is there a bedside tool that provides an indicator of nasal high flow outcomes?
QHow quickly does nasal high flow work?
QIs Optiflow a ventilator?
QIs Optiflow NHF therapy a form of non-invasive (NIV) respiratory support?
QIs the Airvo 2 a continuous positive airway pressure (CPAP) device?
QHow does nasal high flow compare to conventional oxygen therapy (COT)?
QDoes Optiflow NHF therapy provide positive pressure/PEEP?
QWhat clinical evidence is there to support the use of nasal high flow?
QWho will benefit from nasal high flow?
QCan nasal high flow be used in the emergency room (ER)/department (ED)?
QHow should I monitor patients on nasal high flow?
QWhen should I consider escalating patients on nasal high flow?
QHow much pressure is generated by nasal high flow?
QWhat effect does low humidity have on the airway?
PM-622638_a(2020) & PM-624588_a(Jul21)